What Intermolecular Force Would Affect Melting Point the Most
Which intermolecular force would affect melting point the least. Dipole-dipole attractions O B.
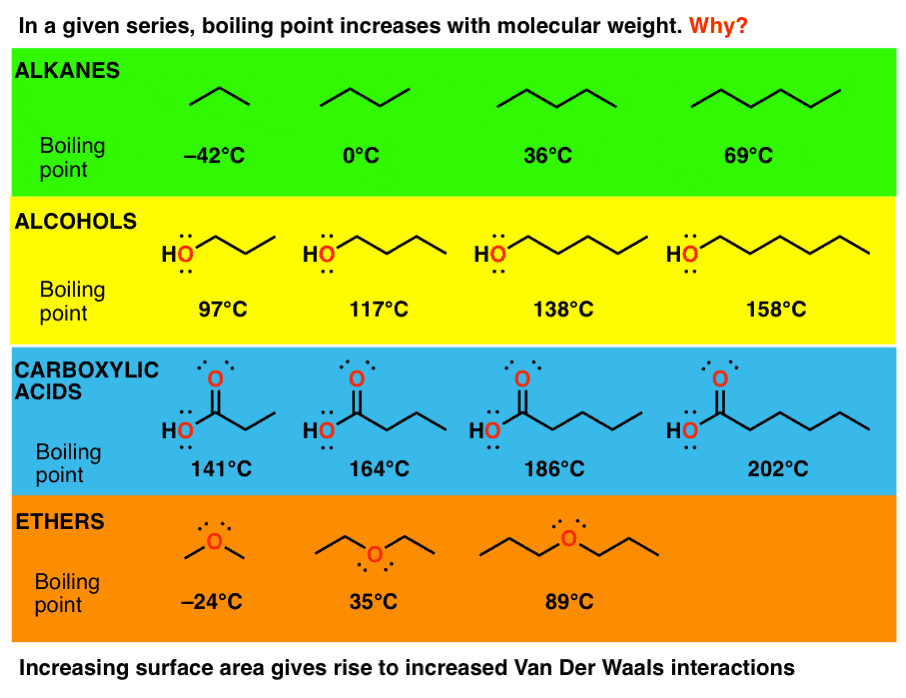
3 Trends That Affect Boiling Points Master Organic Chemistry
2 Points Which intermolecular force would affect melting point the most.
. Many intermolecular forces depend on how strongly atoms in the molecule attract electrons or their electronegativity. Our chief focus up to this point has been to discover and describe the ways in which atoms bond together to form molecules. The stronger the intermolecular forces between the molecules of a liquid the greater the energy required to separate the molecules and turn them into gas à higher boiling point Trends.
Intermolecular attractive forces hold molecules together in the liquid state. Intermolecular forces affect the boiling and melting points of substances. Hydrogen bonding dipole-dipole interactions and ionic interactions represent the three intermolecular forces that have the greatest impact upon the.
Ionic compounds usually have high melting points because the electrostatic forces holding the ions ion-ion interaction are much stronger. The similarity between melting points and boiling points means that the same factors that impact the melting point of a compound will also impact the boiling point. Van der Waals forces C.
Intermolecular Forces Boiling and Melting Points The molecule is the smallest observable group of uniquely bonded atoms that represent the composition configuration and characteristics of a pure compound. Substances with weak intermolecular forces will have low melting and boiling points as less energy heat is needed to overcome these forces. Hydrogen bonding O C.
The presence of polar and especially hydrogen-bonding groups on organic compounds generally leads to higher melting points. Which intermolecular force would affect melting point the most. Those with strong intermolecular forces will have high melting and boiling points as more energy heat is required to overcome.
Nitrogen oxygen fluorine and chlorine are highly electronegative while carbon hydrogen and sulfur are only moderately electronegative. Molecular shape and the ability of a molecule to pack tightly into a crystal lattice has a very large effect on melting points. Therefore the strength and types of intermolecular forces that are found within.
Stronger intermolecular interactions result in higher melting points. Effect on Boiling Point Main Idea. The stronger the intermolecular forces are the more energy is required so the higher the melting point is.
The force of attraction between the molecules affects the melting point of a compound. Which intermolecular force would affect melting point the least Ionic bonds Van der Waals forces hydrogen bonding or Dipole dipole interactions. Van der Waals -.

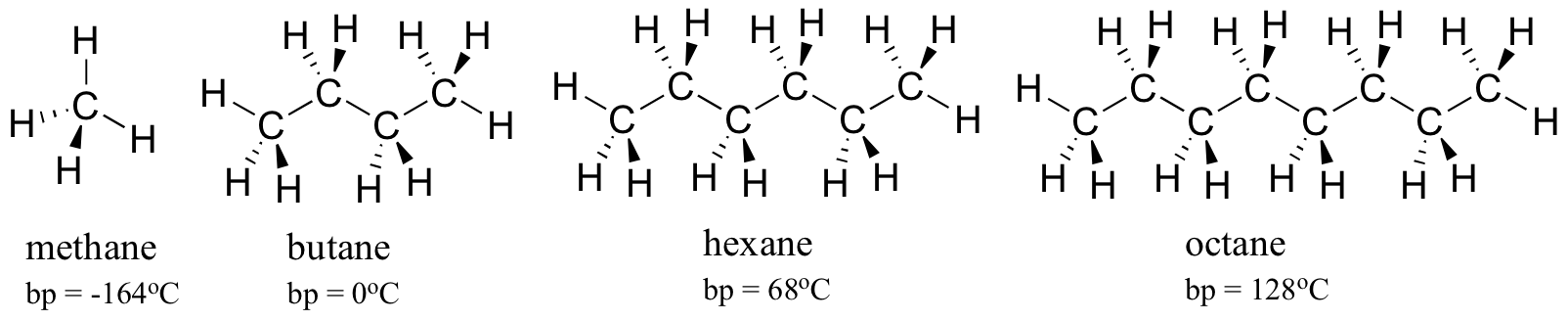
3 Trends That Affect Boiling Points Master Organic Chemistry

Intermolecular Forces Boiling Melting Points Video Lesson Transcript Study Com
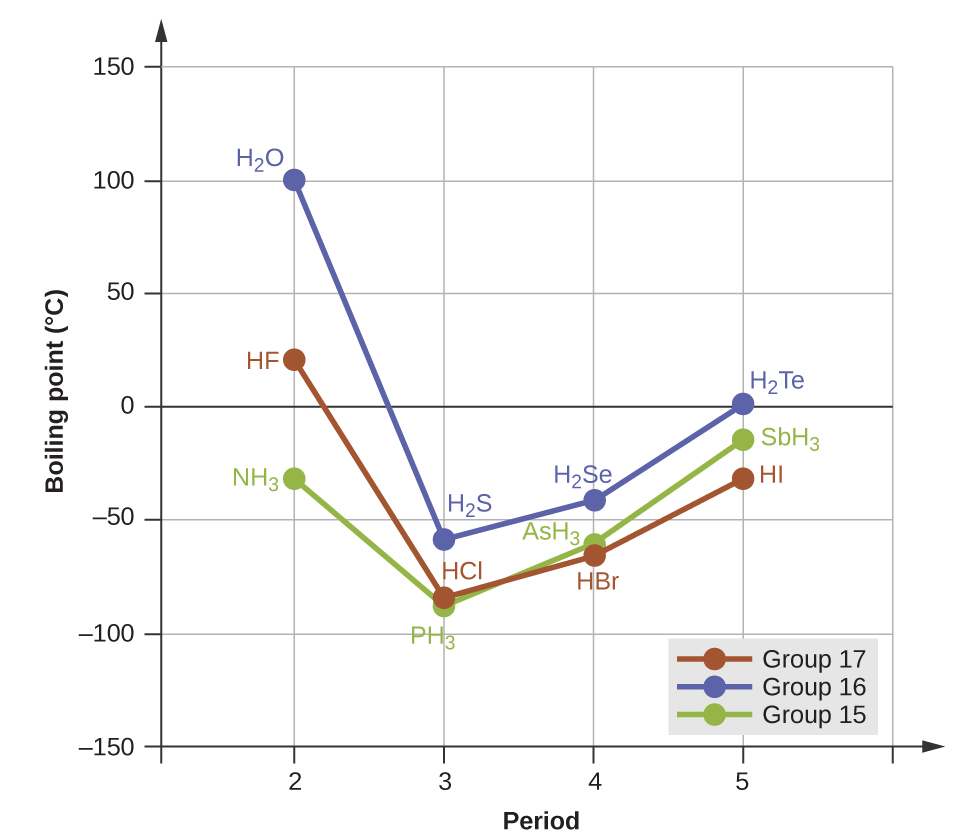
10 1 Intermolecular Forces Chemistry

Intermolecular Forces And Vapor Pressure Ap Chemistry Khan Academy Youtube

Intermolecular Forces Boiling Melting Points Video Lesson Transcript Study Com
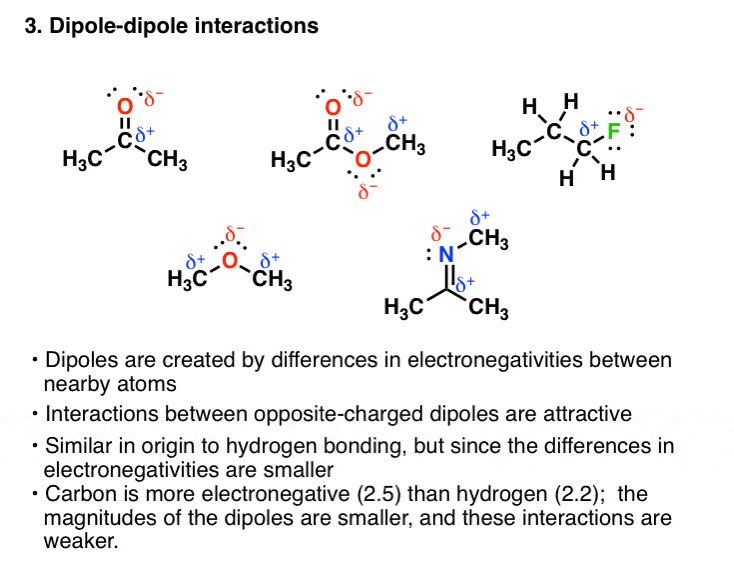
The Four Intermolecular Forces And How They Affect Boiling Points
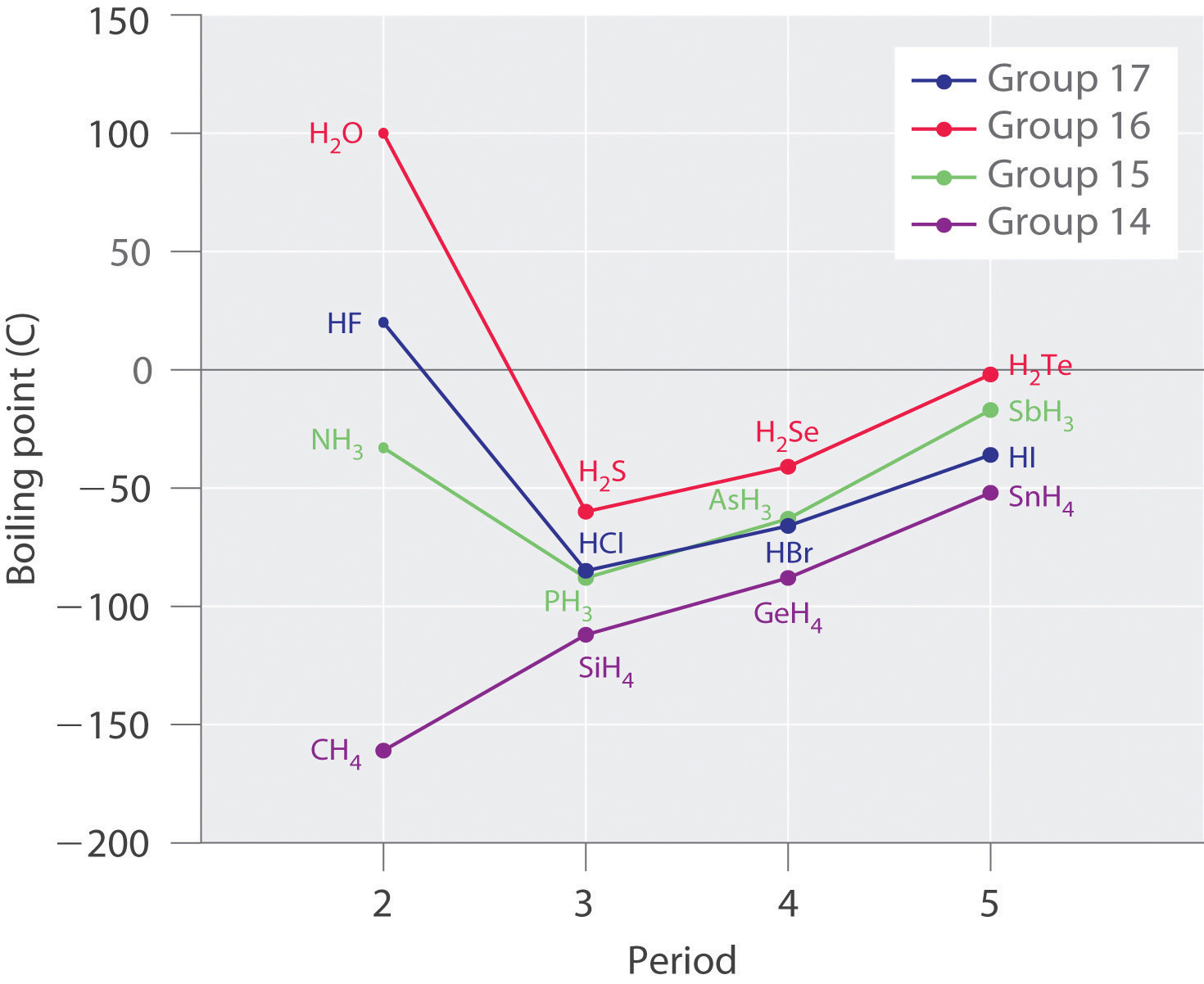
12 6 Types Of Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts

10 1 Intermolecular Forces Chemistry
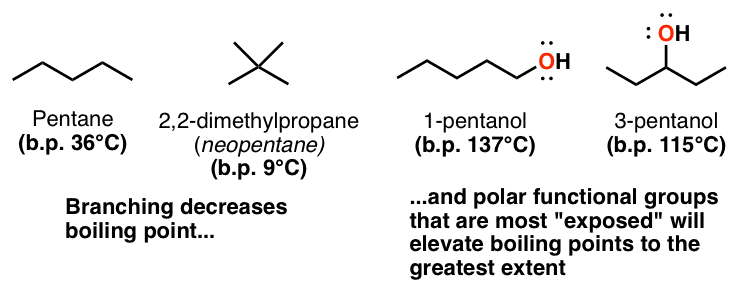
3 Trends That Affect Boiling Points Master Organic Chemistry
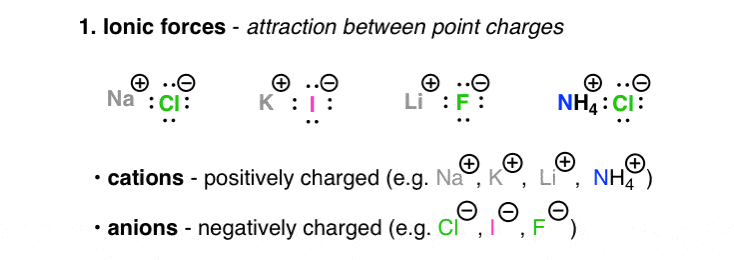
The Four Intermolecular Forces And How They Affect Boiling Points
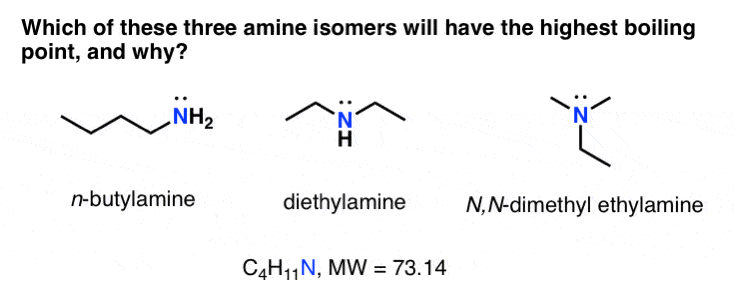
3 Trends That Affect Boiling Points Master Organic Chemistry
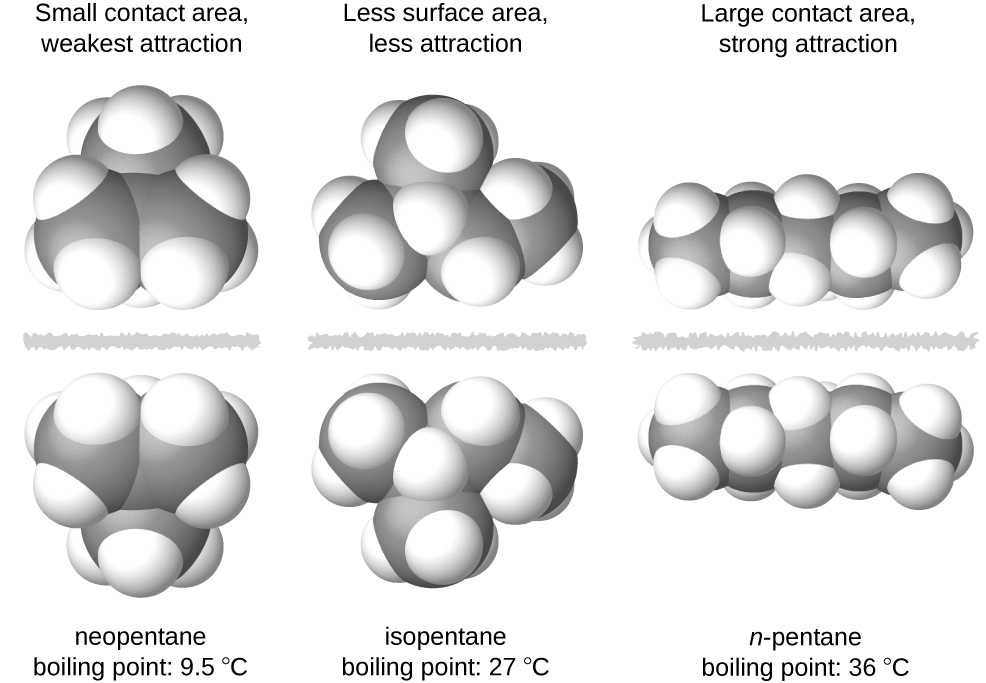
10 1 Intermolecular Forces Chemistry
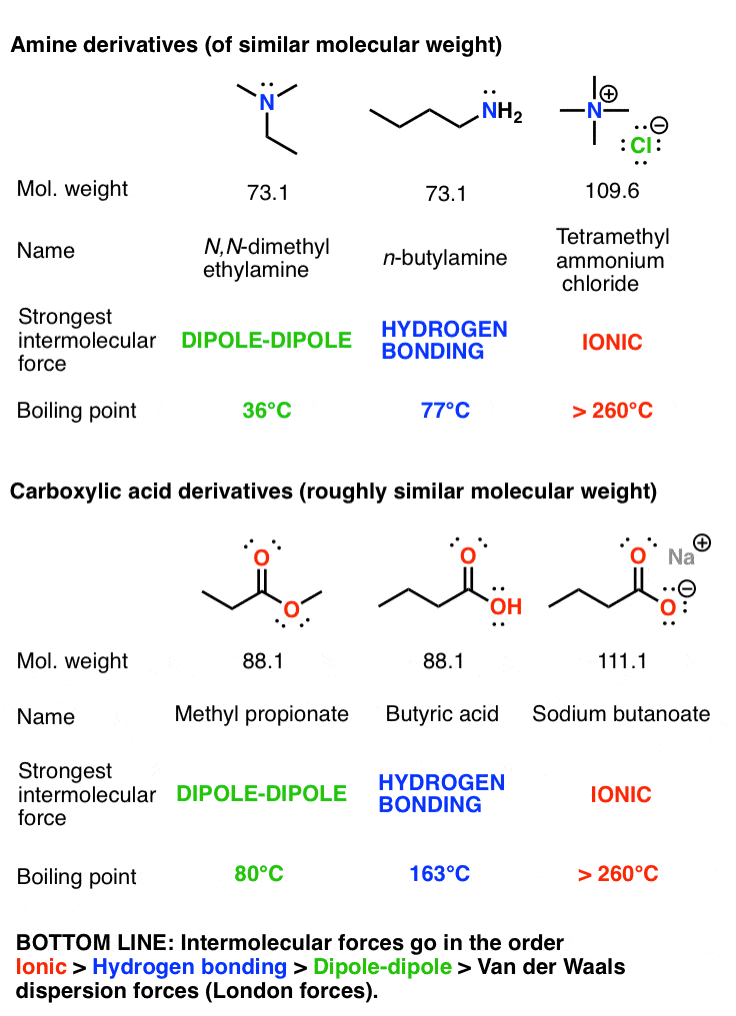
3 Trends That Affect Boiling Points Master Organic Chemistry

12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts

Intermolecular Forces Miscibility Melting And Boiling Points Chemistry Jove

Which Intermolecular Force Would Affect The Boiling Point The Least Brainly Com
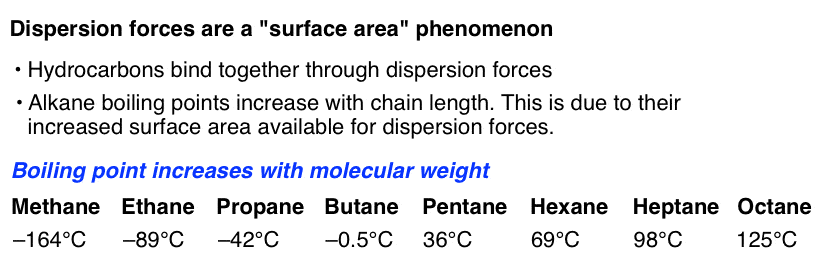
The Four Intermolecular Forces And How They Affect Boiling Points

2 11 Intermolecular Forces And Relative Boiling Points Bp Chemistry Libretexts
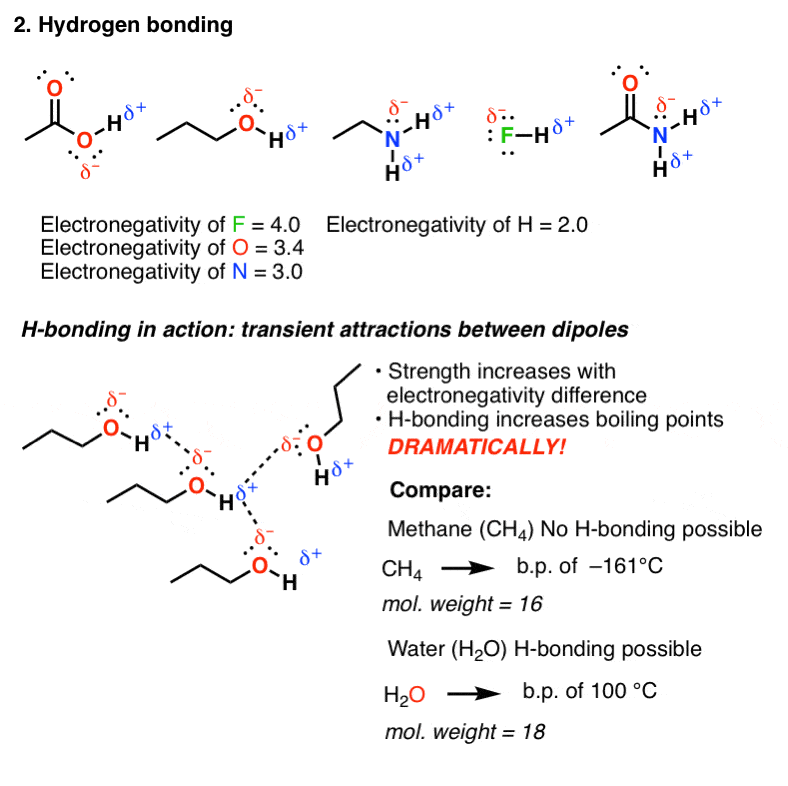
The Four Intermolecular Forces And How They Affect Boiling Points

Comments
Post a Comment